Translate
Thursday, August 15, 2013
Monopoly
I decided to play the Monopoly game the other day, but I wanted to change the rules slightly.
1) An extra person can play the "role" of the bank.
2) Every time a player passes GO, they don't get paid, they have to pay 10% of their current money plus 10% of their original loan in taxes to the Bank. Income only comes from property ownership. Everytime someone lands on your property, they pay.
3) The bank player doesn't move around the board, the bank just loans money and collects it back with interest.
So, after 4 friends played for awhile, it was down to 2 players that dominated. After a little while longer, it was finally down to one regular player and me (the Bank). So, the player kept going around always paying 10% each time he passed GO, but not collecting any more money because there was nobody left to stay at his properties.
After another 45 minutes, he had no money to pay the bank, so a property was repossessed. This went on for another hour until all properties were owned by me and I won.
But how else could the story have ended? All money in existence was debt owed to the central bank....plus interest. But the interest was never printed, so everyone defaults in the end. It is a mathematical certainty.
The best way to win the game is not to play!
Richard
Fukishima update - Can I eat some Salmon?
My last post on Fukishima, seen here, was back in 2011. I made the statement that North America had nothing to worry about, yet and that it was primarily a Japan problem (I feel very sad for what Japan is going through). I still stand by that statement even though some things have changed.
Firstly, the airborne releases are minimal and not even detectable 6,000 miles away on the west coast of Canada and the USA. That is good.
Secondly, we find out that TEPCO has been leaking 300 tons of contaminated water per day into the Pacific. So, my main question...Is it safe to eat Pacific Salmon in BC.
Well, common sense says that Cessium 137 (Cs-137) and Strontium 90 (Sr-90) should sink in the water. I found a reference to that scenario here. It states:
A swift deep current along the coast of Japan is expected to pull the low concentration of radioactive particles from the Fukushima plant to a depth of about 300 feet and dilute it all by a factor of 50 to 100, researchers said.
The prevailing currents then carry the material out to sea, away from beaches and inhabited coasts.
Swept out to sea, depending on the material, many radioactive isotopes such as cesium-137 and strontium-90 usually sink and then remain suspended at depth in the ocean water, sometimes for decades, but have little direct effect on salt-water fish, scientists said.
I know that Cs-137 doesn't bio accumulate in marine life and I was more worried about Sr-90 because it is absorbed into the bones and any calcium rich organs and stays there. After some research, I found this study, Strontium-90 in fish from the lakes of the Chernobyl Exclusion Zone.
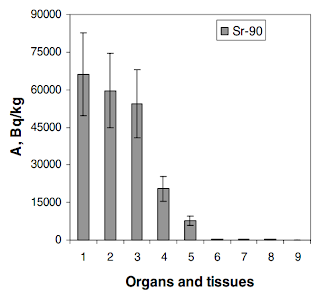
It goes into lots of detail, but the main idea can be expressed in the following graph.
Sr-90 content (specific activity) in organs and tissues of Common crucian carp (Glubokoye Lake):
1 – scales; 2 – bones; 3 – fins; 4 – head; 5 – stomach contents; 6 – skin; 7 – muscles; 8 – internal organs; 9 – roe.
Notice that the scales, bones, fins and head absorb most of the Sr-90, but the muscles absorb almost nothing. Since, we eat the muscles usually, this is a good thing. Also, 70-80% of ingested Sr-90 is eliminated from the fish and also from humans.
This data mostly made me feel better, but I still wanted to see some real world testing. I found an independent test performed last year from a couple living in Seattle, WA. They were testing for Cs-137, but since both Cs-137 and Sr-90 are being constantly leaked from Fukishima, if you don't detect one in a fish, then it stands to reason that the other isn't present in that fish as well. Here is what they got back from the lab.
----------------
Sample 1
Cs-134: < 0.902 Bq/kg (minimum detectable concetrations)
Cs-137: < 1.47 Bq/kg (minimum detectable concetrations)
Sample 2
Cs-134: < 0.891 Bq/kg (minimum detectable concetrations)
Cs-137: < 1.26 Bq/kg (minimum detectable concetrations)
EACH SAMPLE WAS MEASURED FOR 40,000 SECONDS ON A HIGH PURITY GERANIUM DETECTOR
The units Bq/kg are Becquerels per kilogram, a measurement of decay rate of an isotope. One Becquerel is another way of saying "one nuclear decay per second."
For comparison, the typical activity concentration of the naturally-occurring isotope Potassium-40 (K-40) in salmon is on the order of 100 Bq/kg, or 100 decays per second per kilogram. So they showed that the activity levels of Cs-134 and Cs-137 cannot be more than about 1% of the K-40 level, which is a very low level.
-----------------
So, we are good to go on wild Pacific Salmon, but I wouldn't want seafood shipped in from Japan. And we still have the leak going on. I also worry about the next phase of cleanup in which spent rods have to be manually moved from a cooling pond. We'll just have to keep watching this.
Firstly, the airborne releases are minimal and not even detectable 6,000 miles away on the west coast of Canada and the USA. That is good.
Secondly, we find out that TEPCO has been leaking 300 tons of contaminated water per day into the Pacific. So, my main question...Is it safe to eat Pacific Salmon in BC.
Well, common sense says that Cessium 137 (Cs-137) and Strontium 90 (Sr-90) should sink in the water. I found a reference to that scenario here. It states:
A swift deep current along the coast of Japan is expected to pull the low concentration of radioactive particles from the Fukushima plant to a depth of about 300 feet and dilute it all by a factor of 50 to 100, researchers said.
The prevailing currents then carry the material out to sea, away from beaches and inhabited coasts.
Swept out to sea, depending on the material, many radioactive isotopes such as cesium-137 and strontium-90 usually sink and then remain suspended at depth in the ocean water, sometimes for decades, but have little direct effect on salt-water fish, scientists said.
I know that Cs-137 doesn't bio accumulate in marine life and I was more worried about Sr-90 because it is absorbed into the bones and any calcium rich organs and stays there. After some research, I found this study, Strontium-90 in fish from the lakes of the Chernobyl Exclusion Zone.
It goes into lots of detail, but the main idea can be expressed in the following graph.
Sr-90 content (specific activity) in organs and tissues of Common crucian carp (Glubokoye Lake):
1 – scales; 2 – bones; 3 – fins; 4 – head; 5 – stomach contents; 6 – skin; 7 – muscles; 8 – internal organs; 9 – roe.
Notice that the scales, bones, fins and head absorb most of the Sr-90, but the muscles absorb almost nothing. Since, we eat the muscles usually, this is a good thing. Also, 70-80% of ingested Sr-90 is eliminated from the fish and also from humans.
This data mostly made me feel better, but I still wanted to see some real world testing. I found an independent test performed last year from a couple living in Seattle, WA. They were testing for Cs-137, but since both Cs-137 and Sr-90 are being constantly leaked from Fukishima, if you don't detect one in a fish, then it stands to reason that the other isn't present in that fish as well. Here is what they got back from the lab.
----------------
Sample 1
Cs-134: < 0.902 Bq/kg (minimum detectable concetrations)
Cs-137: < 1.47 Bq/kg (minimum detectable concetrations)
Sample 2
Cs-134: < 0.891 Bq/kg (minimum detectable concetrations)
Cs-137: < 1.26 Bq/kg (minimum detectable concetrations)
EACH SAMPLE WAS MEASURED FOR 40,000 SECONDS ON A HIGH PURITY GERANIUM DETECTOR
The units Bq/kg are Becquerels per kilogram, a measurement of decay rate of an isotope. One Becquerel is another way of saying "one nuclear decay per second."
For comparison, the typical activity concentration of the naturally-occurring isotope Potassium-40 (K-40) in salmon is on the order of 100 Bq/kg, or 100 decays per second per kilogram. So they showed that the activity levels of Cs-134 and Cs-137 cannot be more than about 1% of the K-40 level, which is a very low level.
-----------------
So, we are good to go on wild Pacific Salmon, but I wouldn't want seafood shipped in from Japan. And we still have the leak going on. I also worry about the next phase of cleanup in which spent rods have to be manually moved from a cooling pond. We'll just have to keep watching this.
Subscribe to:
Comments (Atom)